2 years ago
narzafaydu
Chemical Reactions
Chemical reactions happen absolutely everywhere. While we sometimes associate chemical reactions with the sterile environment of the test tube and the laboratory - nothing could be further from the truth. In fact, the colossal number of transformations make for a dizzying, almost incomprehensible array of new substances and energy changes that take place in our world every second of every day.
In nature, chemical reactions can be much less controlled than you’ll find in the lab, sometimes far messier, and they generally occur whether you want them to or not! Whether it be a fire raging across a forest (Figure 1), the slow process of iron rusting in the presence of oxygen and water over a period of years, or the delicate way in which fruit ripens on a tree, the process of converting one set of chemical substances (the reactants) to another set of substances (the products) is one known as a chemical reaction.

Though chemical reactions have been occurring on Earth since the beginning of time, it wasn’t until the 18th century that the early chemists started to understand them. Processes like fermentation, in which sugars are chemically converted into alcohol, have been known for centuries; however, the chemical basis of the reaction was not understood. What were these transformations and how were they controlled? These questions could only be answered when the transition from alchemy to chemistry as a quantitative and experimental science took place.
Historical context
Beginning in the early Middle Ages, European and Persian philosophers became fascinated with the way that some substances seemed to “transmute” (or transform) into others. Simple stones, such as those that contained sulfur, seemed to magically burn; and otherwise unimpressive minerals were transformed, like the ore cinnabar becoming an enchanting silvery liquid metal mercury when heated. Alchemists based their approach on Aristotle’s ideas that everything in the world was composed of four fundamental substances - air, earth, fire, and water (Figure 2).
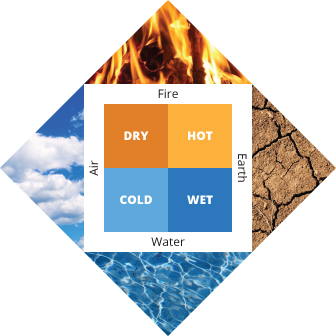
As such, they proposed, and spent generations trying to prove, that less expensive metals like copper and mercury could be turned into gold. Despite their misguided approach, many early alchemists performed foundational chemical experiments - transforming one substance into another, and so it is difficult to point to a specific date or event as the birth of the idea of an ordered, quantifiable chemical reaction. However, there are some important moments in history that have helped to make sense of it.
Lavoisier: Law of Mass Conservation
Antoine Lavoisier was a French nobleman in the 1700s who began to experiment with different chemical reactions. At the time, chemistry still couldn’t be described as being a true, quantitative science. Most of the theories that existed to explain the way that substances changed relied upon Greek philosophy, and there was precious little experimental detail attached to the alchemist’s tinkering.
However, during the second half of the 18th century, Lavoisier performed many quantitative experiments and observed that while substances changed form during a chemical reaction, the mass of the system – or a measure of the total amount of “stuff” present – did not change. In doing so, Lavoisier championed the idea of conservation of mass during transformations (Figure 3). In other words, unlike the alchemists before him who thought that they were creating matter out of nothing, Lavoisier proposed that substances are neither created nor destroyed, but rather change form during reactions. Lavoisier’s ideas were published in the seminal work Traité élémentaire de Chimie in 1789 (Lavoisier, 1789), which is widely hailed as the birth of modern chemistry as a quantitative science.

Proust: Law of Constant Composition
Joseph Proust was a French actor who followed in Lavoisier’s footsteps. Proust performed dozens of chemical reactions, starting with different amounts of various materials. Over time he observed that no matter how he started a certain chemical reaction, the ratio in which the reactants were consumed was always constant. For example, he worked extensively with copper carbonate and no matter how he changed the ratio of starting reactants, the copper, carbon, and oxygen all reacted together in a constant ratio (Proust, 1804). As a result, in the last few years of the 18th century, Proust formulated the law of constant composition (also referred to as the law of definite proportions, Figure 4).
He realized that any given chemical substance (that we now define as a compound) always consisted of the same ratio by mass of its elemental parts regardless of the method of preparation. This was a huge step forward in modern chemistry since it had been previously believed that the substances formed during chemical reactions were random and disordered.

Dalton: Law of Multiple Proportions
The English chemist John Dalton helped make sense of the laws of conservation of mass and definite proportions in 1803 by proposing that matter was made of atoms of unique substances that could not be created or destroyed (see our module Early Ideas about Matter for more information).
Dalton extended Proust’s ideas by recognizing that it was possible for two elements to form more than one compound, but that whatever the compound was, it would always contain elements combined in whole number ratios (Dalton, 1808). This observation is known as the law of multiple proportions (Figure 5) and with his atomic theory, helped to cement Lavoisier’s observations.

These advancements, taken together, laid the groundwork for our modern understanding of chemical reactions, chemical equations, and chemical stoichiometry, or the process of expressing the relative quantities of reactants and products in a chemical reaction.
Comprehension Checkpoint
____ first theorized that while substances changed form during a chemical reaction, the mass of the system did not change.
Types of chemical reactions
There is a staggering array of chemical reactions. Chemical reactions occur constantly within our bodies, within plants and animals, in the air that circulates around us, in the lakes and oceans that we swim in, and even in the soil where we grow crops and build our homes. In fact, there are so many chemical reactions that occur that it would be difficult, if not impossible, to understand them all. However, one method that helps us to understand them is to categorize chemical reactions into a few, general types. While not a perfect system, placing reactions together according to their similarities helps us to identify patterns, which in turn allows predictions to be made about as yet unstudied reactions. In this module, we will consider and provide some context for a few categories of reactions, specifically: synthesis, decomposition, single replacement, double replacement, REDOX (including combustion), and acid-base reactions.
No matter the type of reaction, one universal truth applies to all chemical reactions. For a process to be classified as a chemical reaction, i.e., one where a chemical change takes place, a new substance must be produced. The formation of a new substance is nearly always accompanied by an energy change, and often with some kind of physical or observable change. The physical change can be of different types, such as the formation of bubbles of a gas, a solid precipitate, or a color change. These changes are clues to the existence of a chemical reaction and are important triggers for further research by chemists.
Synthesis reactions
Prior to Lavoisier’s work, it was poorly understood that there were different gases made up of different elements. Instead, various gases were commonly mischaracterized as types of "air" or air missing parts – for example, terms commonly used were "inflammable air," or "dephlogisticated air." Lavoisier thought differently and was convinced that these were different substances. He conducted experiments where he mixed inflammable air with dephlogisticated air and a spark, and he found that the substances combined to produce water. In response, he renamed inflammable air "hydrogen" from the Greek hydro for "water" and genes for "creator." In so doing, Lavoisier was identifying a synthesis reaction. In general, a synthesis reaction is one in which simpler substances combine to form another more complex one. Hydrogen and oxygen (which Lavoisier also renamed dephlogisticated air) combine in the presence of a spark to form water, summarized by the chemical equation shown below (for more on chemical equations see the section called Anatomy of a chemical equation), it represents a simple synthesis reaction.
2 years ago
narzafaydu
Natural eye cream products are becoming more and more indispensable in our skin care regimen. Find out if this is a better choice over seemingly more powerful chemical-based products. https://www.novatismed.co
